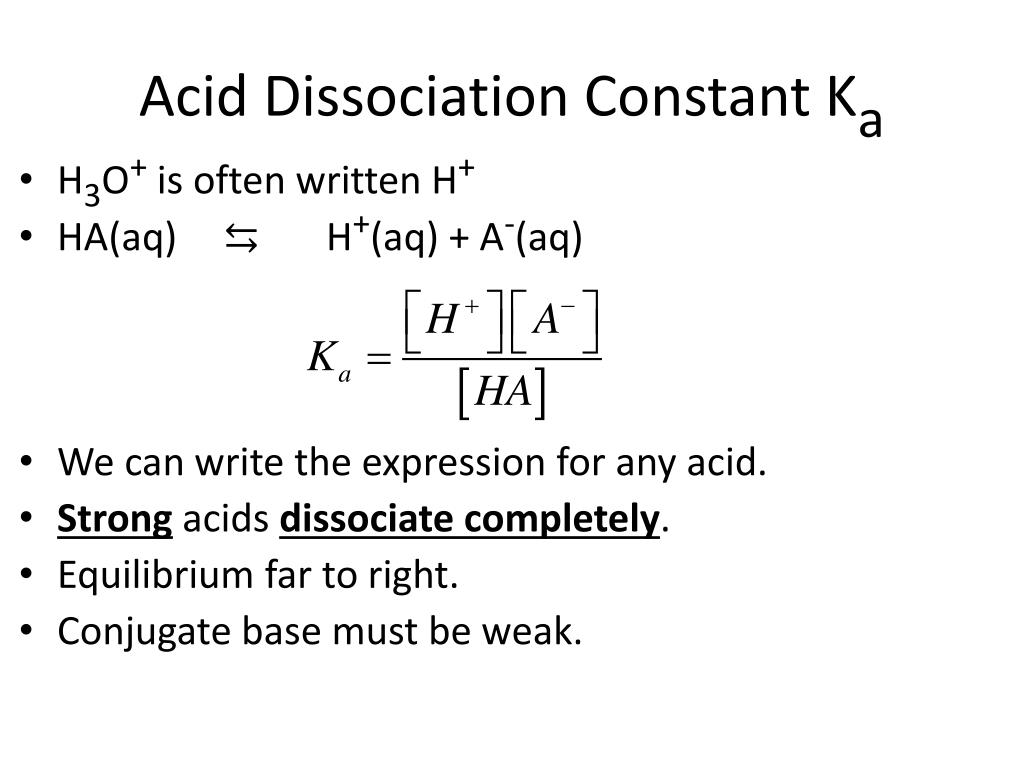
High Dissociation Constant Means . if the reaction has a weak conjugate acid and a strong reactant acid, the equilibrium constant will be high. what is the dissociation constant (kd)? the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. Ka = [h3o +][a −] [ha]. for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on; Association constant (k a or k a ) is the. This means that the reaction. the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant:
from www.slideserve.com
the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. Association constant (k a or k a ) is the. the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: Ka = [h3o +][a −] [ha]. what is the dissociation constant (kd)? the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on; This means that the reaction. if the reaction has a weak conjugate acid and a strong reactant acid, the equilibrium constant will be high.
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download
High Dissociation Constant Means the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. This means that the reaction. for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: Association constant (k a or k a ) is the. the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. if the reaction has a weak conjugate acid and a strong reactant acid, the equilibrium constant will be high. what is the dissociation constant (kd)? the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. Ka = [h3o +][a −] [ha]. dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on;
From www.slideserve.com
PPT Thermodynamics and PowerPoint Presentation, free High Dissociation Constant Means dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on; the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. the equilibrium constant for this reaction is the acid ionization constant ka, also called. High Dissociation Constant Means.
From byjus.com
37. Among the following the dissociation constant is highest for (1 High Dissociation Constant Means the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. Ka = [h3o +][a −] [ha]. what is the dissociation constant (kd)? Association constant (k a or k a ) is the. for an aqueous solution of a weak acid, the dissociation constant is called the. High Dissociation Constant Means.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download High Dissociation Constant Means This means that the reaction. the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. Association constant (k a or k a ) is the. Ka = [h3o +][a −] [ha]. for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization. High Dissociation Constant Means.
From www.slideserve.com
PPT Arrhenius Definition PowerPoint Presentation, free download ID High Dissociation Constant Means what is the dissociation constant (kd)? Association constant (k a or k a ) is the. for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. the. High Dissociation Constant Means.
From www.ck12.org
Dissociation Constant (K[w]) Example 1 ( Video ) Chemistry CK12 High Dissociation Constant Means This means that the reaction. the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). what is the dissociation constant (kd)? dissociation constant (k d ) is the rate constant. High Dissociation Constant Means.
From dxowmggun.blob.core.windows.net
Higher Dissociation Constant Means at Thomas Partin blog High Dissociation Constant Means what is the dissociation constant (kd)? Association constant (k a or k a ) is the. This means that the reaction. the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. Ka = [h3o +][a −] [ha]. dissociation constant (k d ) is the rate constant. High Dissociation Constant Means.
From slidetodoc.com
Experiment 9 Determination of Dissociation Equilibrium Constant of High Dissociation Constant Means Association constant (k a or k a ) is the. the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. Ka = [h3o +][a −] [ha]. the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: the dissociation. High Dissociation Constant Means.
From learnaboutpharma.com
Dissociation constant Definition, Explanation, Formula, Example, and High Dissociation Constant Means dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on; for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). Ka = [h3o +][a −] [ha]. what is the dissociation constant (kd)? the equilibrium constant for. High Dissociation Constant Means.
From sciencenotes.org
Dissociation (Chemistry) Definition and Examples High Dissociation Constant Means This means that the reaction. the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. if the reaction has a weak conjugate acid and a strong reactant acid, the equilibrium constant will be high. dissociation constant (k d ) is the rate constant of dissociation at. High Dissociation Constant Means.
From www.youtube.com
Strong Acid Ka Dissociation Constant Chemistry (HCl, HBr, HI, HNO3 High Dissociation Constant Means the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. Association constant (k a or k a ) is the. what is the dissociation constant (kd)? for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). This means. High Dissociation Constant Means.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download High Dissociation Constant Means the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k. High Dissociation Constant Means.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID6776086 High Dissociation Constant Means the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: Association constant (k a or k a ) is the. Ka = [h3o +][a −] [ha]. dissociation constant. High Dissociation Constant Means.
From www.youtube.com
Correct relation between dissociation constants of a dibasic acid High Dissociation Constant Means dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on; the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant. High Dissociation Constant Means.
From learnaboutpharma.com
Dissociation constant Definition, Explanation, Formula, Example, and High Dissociation Constant Means This means that the reaction. what is the dissociation constant (kd)? the dissociation constant specifies the tendency of a substance m x n y to reversibly dissociate (separate) in a solution (often. Association constant (k a or k a ) is the. the dissociation constant (kd) is a quantitative measure of the affinity between a protein and. High Dissociation Constant Means.
From www.sliderbase.com
Acids, pH and Equilibrium Presentation Chemistry High Dissociation Constant Means the dissociation constant (kd) is a quantitative measure of the affinity between a protein and its ligand, representing the concentration of. for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k. High Dissociation Constant Means.
From www.researchgate.net
Dissociation constant (KD) values ± SE measured by equilibrium dialysis High Dissociation Constant Means Association constant (k a or k a ) is the. This means that the reaction. the equilibrium constant for this reaction is the acid ionization constant ka, also called the acid dissociation constant: Ka = [h3o +][a −] [ha]. if the reaction has a weak conjugate acid and a strong reactant acid, the equilibrium constant will be high.. High Dissociation Constant Means.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID269665 High Dissociation Constant Means dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on; Ka = [h3o +][a −] [ha]. for an aqueous solution of a weak acid, the dissociation constant is called the acid ionization constant (\(k_a\)). if the reaction has a weak conjugate acid and a strong. High Dissociation Constant Means.
From www.researchgate.net
shows the qualitatively correct variation of the dissociation constant High Dissociation Constant Means what is the dissociation constant (kd)? if the reaction has a weak conjugate acid and a strong reactant acid, the equilibrium constant will be high. Ka = [h3o +][a −] [ha]. dissociation constant (k d ) is the rate constant of dissociation at equilibrium, defined as the ratio k off / k on; the dissociation constant. High Dissociation Constant Means.